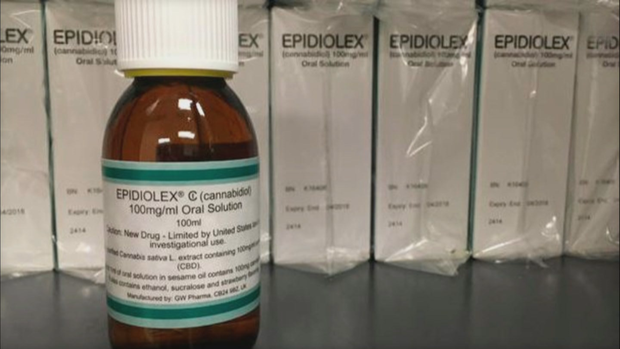
First Marijuana-Based Drug Gets Mixed Reactions In Colorado
By Kathy Walsh
COLORADO SPRINGS, Colo. (CBS4) - The Federal and Drug Administration has approved the country's first drug derived from marijuana, a medication to treat two rare and devastating forms of epilepsy.
This milestone is expected to help patients ages 2 and older with Dravet and Lennox-Gastaut syndromes. But some parents worry it could hurt Colorado's cannabidiol (CBD) industry already helping children who are suffering from seizures.
Years ago, the Stanley brothers of southern Colorado grew a strain of marijuana low in THC and high in CBD. Heather Jackson of Colorado Springs gave it to her son.
"CBD oil for my son was life. He wouldn't be here without that," said Jackson.
Zaki Jackson is 15 years old now and living with a rare form of epilepsy called Doose syndrome. At just 4 months old, he started having violent seizures.
"He would average 200 a day," said Jackson.
She says 17 doctor-prescribed drugs didn't help her son. But six years ago, she started giving him oil, potent with CBD, made from a strain of marijuana grown in Teller County by the Stanley brothers. It was low in THC, what gets you high, and it was hope for Zaki.
"CBD put my son's condition into remission," Jackson told CBS4 Health Specialist Kathy Walsh.
Jackson is now CEO of the Realm of Caring, a 501(c)(3) not-for-profit organization which provides support services for individuals using cannabinoid therapy. She is cautiously optimistic about FDA approval of Epidiolex, the first drug made of CBD.
"There's a drug route and a pharmaceutical route and there's a dietary supplement route and we need to have both of those options available," said Jackson.
But Paige Figi is worried.
"They're going to try and shut down the US market that already exists," she said.
Figi's daughter, Charlotte, was the first to use the Colorado CBD. The plant was renamed 'Charlotte's Web'.
Figi is now executive director of Coalition for Access Now, a political nonprofit trying to make CBD as legal as a dietary supplement. She's concerned about the British company, GW Pharmaceuticals, behind the new drug.
"That lobbies heavily against any reform except for their one drug," Figi said.
Heather Jackson believes if her son, Zaki, couldn't take his CBD he would die.
"That can't happen," she said choking back tears.
In a statement, Joel Stanley, who now markets and sells Charlotte's Web CBD (CW Hemp), said:
We think this is great news and it doesn't change anything for the hemp-based dietary supplement industry. It does show that the perceptions and the true understanding of cannabinoid related health is changing dramatically. We are hopeful that the new GW drug will help many people with the two diseases it is approved for and for whom insurance will cover the drug. Unfortunately, as with most pharmaceutical drugs, we have all seen that no one drug can act as a panacea against such devastating conditions. Nevertheless, we're encouraged that the Epidiolex approval will bring more and more research interest and funding to cannabinoid based therapies that have the potential to provide significant changes to consumers' lives.
Kathy Walsh is CBS4's Weekend Anchor and Health Specialist. She has been with CBS4 since 1984. She is always open to story ideas. Follow Kathy on Twitter @WalshCBS4.